Labeling
AI-POWERED AUTOMATION SOLUTION FOR LIFE SCIENCES
Streamline label changes, stay compliant, and gain real-time insights with Process Automation, AI, and Data Fabric.
AI Skills: Conversation
The Labeling solution creates and manages label changes and provides real-time visibility into the global label change process. It empowers individual countries to customize and manage their own workflows without local Appian development expertise using Case Management Studio. It augments your existing systems such as Veeva by leveraging the process automation, AI and Data Fabric capabilities to streamline the label change management from end-to-end.
Watch Solution Demo
Please tell us about yourself

The new version, powered by Appian AI is now available.
Business Drivers

Managing label changes is a strategic business process for pharmaceutical companies, involving complex global workflows and stringent regulatory compliance.
Princeton Blue’s enhanced Labeling solution addresses these challenges while providing greater flexibility and efficiency.
- Global Spread: Management of label change requests that are created and approved at a global level and implemented by teams spread across multiple countries.
- Country Specific Workflows: Each country follows its own guidelines for submitting the label change that needs to be incorporated into the process.
- Dependency management: Managing the impact of an approved label version on other in-flight label changes.
- Process Visibility: Real-time transparency into the status progression across the countries including all versions of label changes.
- Access to Documents: Availability of the right documents relating to the Labeling request.
- Statutory SLA Compliance: Accurate monitoring and reporting of SLA compliance.
Watch Solution Demo
Please tell us about yourself
The new version, powered by Appian AI is now available.

Managing Label Changes
The Labeling solution from Princeton Blue augments your existing systems such as Veeva to automate the workflow and orchestrates collaboration for processing label changes swiftly.
The solution leverages Appian’s Case Management Studio to create a controlled, fully auditable, and customizable label change process. It maintains rigorous quality checks and regulatory compliance through multiple review stages, while enabling global teams to customize their workflows to meet their local needs and requirements without any dependence on Headquarters.
This approach automates and orchestrates the entire global labeling process, facilitating seamless collaboration among various stakeholders worldwide, without depending on Headquarters or requiring specialized Appian development skills at the local level.
How the Labeling Solution Works
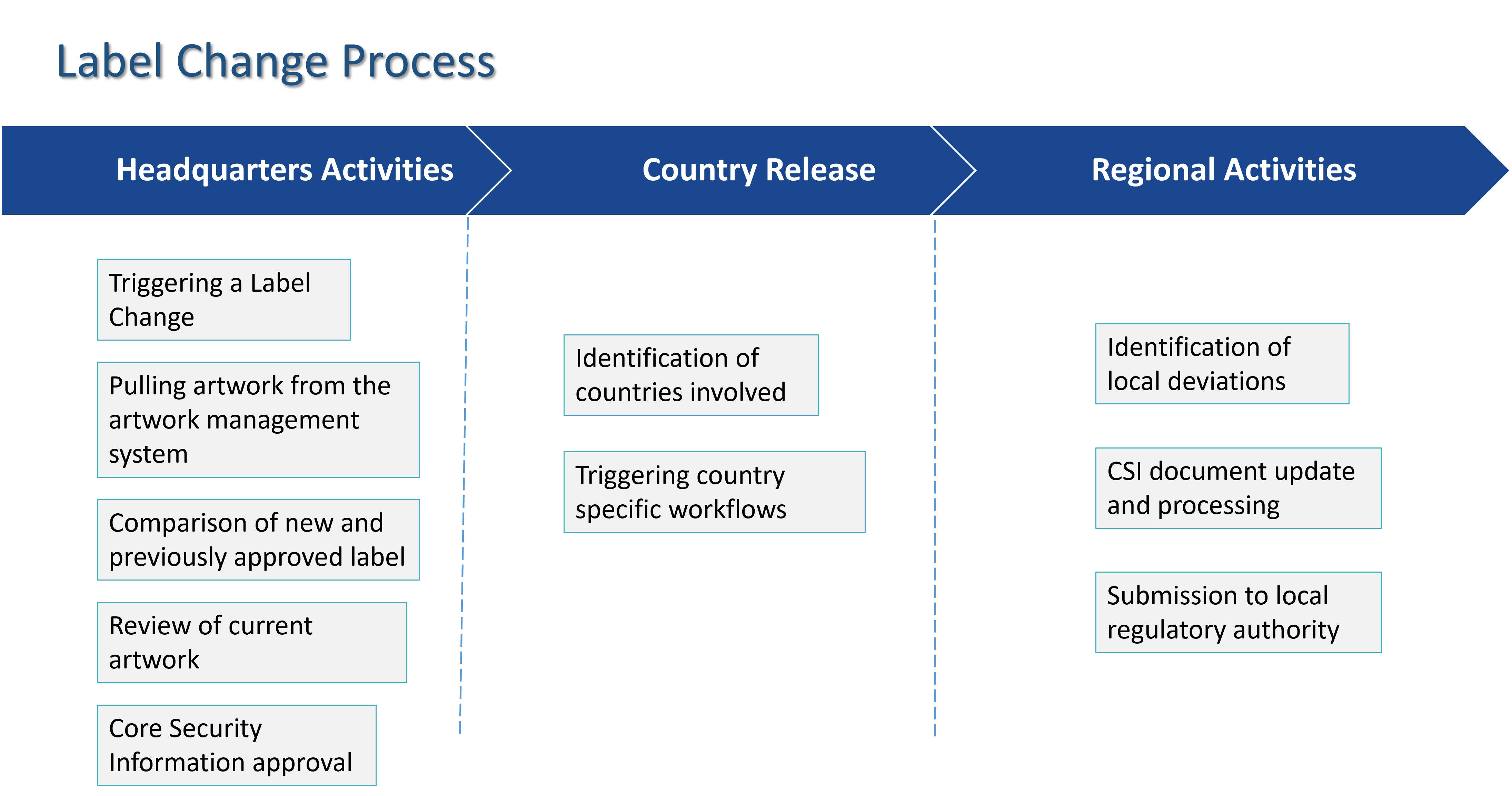
Once a label change is initiated, the Labeling solution routes the change request for review and approval from headquarters and the impacted countries. The solution provides a comparison of the label change request with the latest approved label.
The solution integrates with your Artwork Management system to pull in the necessary artwork and provides a comparison of the new label vs the previously approved label. This provides the required visibility into the changes during the review and approval portion of the process.
Once the label change request has been approved, it is released to the impacted countries via Appian Case Management. As the tasks are performed by the representatives of each country, the status is tracked at a global level for each label change request.
When a label version is approved by the local health agency for a specific country, the label change requestor at headquarters is automatically notified about the approval, so the new label can become the basis for future label changes.
Key Features of the Solution

Process Orchestration

Process Visibility

Country-Specific Workflow Customization

Exception Management

Unified Data View

Document Comparison and Management

Intuitive User Interface
Collaborative Ecosystem

Scalable Architecture

Seamless Regulatory Compliance

AI-Powered
