
Network, exchange ideas, and collaborate with your peers from the pharmaceutical industry.
October 8, 2024
Pfizer, NYC Headquarters
66 Hudson Blvd E, Ste 20
New York, NY 10001

Event Highlights
Join Princeton Blue, Appian, and your fellow innovators in the Life Sciences industry for a day of groundbreaking insights and collaborative learning. Together, we’ll explore:
- The transformative impact of AI and process automation on the life sciences industry
- Strategies to accelerate product development and time-to-market
- Real-world applications that are reshaping patient care and operational efficiency
Why Attend?
- Network with Industry Leaders: Connect with professionals, thought leaders, and tech visionaries at the forefront of life sciences innovation.
- Gain Practical Insights: Learn how to effectively implement AI and process automation to streamline your processes and boost productivity.
- Explore Real-World Success Stories: Hear firsthand accounts of challenges overcome and successes achieved through AI and process automation.
- Stay Ahead of the Curve: Position your organization at the vanguard of the rapidly evolving life sciences landscape.
Don't miss this opportunity to be part of shaping the future of life sciences. Join Princeton Blue and industry peers as we delve into the practical applications of AI and process automation that are revolutionizing our field.
Agenda and Registration
Fresh from the Oven
In line with the theme, we plan to launch two brand new solutions from the Princeton Blue Innovation Lab during the event.
These solutions leverage the latest Gen AI and Case Management capabilities of the Appian Platform.
Labeling
Clinical Complaint Management
Our Experience in Life Sciences
Princeton Blue is a trusted leader in process automation for pharmaceutical and biotech companies using Low-code technology to rapidly improve business performance in a matter of weeks.
We help clients design solutions to augment your existing systems such as Veeva, Argus, Oracle, and LIMS/ELN and present a unified view of your data, integrate with your existing applications, and deliver workflow automation that spans your entire business process.
Princeton Blue is recognized by leading industry analysts for deep expertise in Low-code technologies and decades of work experience in the Life Sciences industry. Some of the world’s largest pharmaceutical and biotech companies trust us to automate their most strategic Lab, Clinical and Regulatory processes.
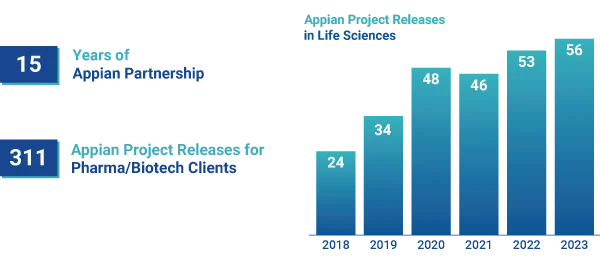
Leverage our Deep Appian Expertise
Princeton Blue has been a trusted Appian partner for 15 years, and during that time, we have delivered over 300 Appian projects for our Pharma and Biotech customers.
Using Low-Code Automation to Manage the Clinical Supply Chain
See how workflow automation and data integration across siloed systems helped Merck drive significant value.
Hear from Merck on Clinical Automation
Mercks shares its automation journey around managing change in Clinical Data Management with Appian and Princeton Blue.
Projects Delivered
Clinical
Protocol Activity Schedule
Patient Eligibility
Clinical Supplies Job Readiness
Clinical Supplies Scheduling Tool
Study and Site Feasibility
Site Contact Information
Real-World Evidence
Monitoring Visit Report
User Access Management
Study Start-up
Clinical Planning
Standards Request Workflow
Patient Engagement Management
Integrated Evidence Planning
Clinical Complaint Management
Clinical Identity Access Management
Manufacturing
Asset Disposal
Temperature Excursion
Regulatory
EU Clinical Trials Regulation
Label Change Workflow
Dossier Change Control
Health Authority Correspondence
Regulatory Submission Planning
Regulatory Information Management
Lab
Lab Experiment Automation
Lifecycle Management
Lab Shared Services Request Processing
Medical Devices
Personalized Surgical Planning and Implementation
Regulatory Information Management
Clinical
Protocol Activity Schedule
Patient Eligibility
Clinical Supplies Job Readiness
Clinical Supplies Scheduling Tool
Study and Site Feasibility
Site Contact Information
Real-World Evidence
Monitoring Visit Report
User Access Management
Study Start-up
Clinical Planning
Standards Request Workflow
Patient Engagement Management
Integrated Evidence Planning
Clinical Complaint Management
Clinical Identity Access Management
Regulatory
EU Clinical Trials Regulation
Label Change Workflow
Dossier Change Control
Health Authority Correspondence
Regulatory Submission Planning
Regulatory Information Management
Lab
Lab Experiment Automation
Lifecycle Management
Lab Shared Services Request Processing
Medical Devices
Personalized Surgical Planning and Implementation
Regulatory Information Management
Appian Solutions for Life Sciences
Clinical Complaint Management
Automate complaint handling, review, and categorization in clinical trials with Appian AI.

Labeling
Automate label management, stay compliant, and gain real-time insights with Process Automation, AI, and Data Fabric.

EU-CTR
Automate your EU-CTR compliance workflows for faster, error-free clinical submissions.

Regulatory Submissions
Streamline your regulatory submissions process for faster approvals and better compliance.

Clinical Supplies Shipping
Automate clinical supplies shipping with AI for consistent, efficient, on-time delivery.

Study Data Access Management
Securely manage user access requests for study data using AI & Process Automation.

Study Start-up
Automate the workflow and collaboration of the clinical study start-up process allowing for variations in business rules and regulatory requirements across countries.

Lab Experiment Automation
Automate lab operations to improve real-time visibility, reduce delays and drive reliable outcomes.
Drug Safety
Automate drug safety workflows for faster, consistent, and audit-ready reporting.

Dynamic Emergency Response
Streamline the triage between hospital nurses, doctors and ambulance EMT when a 911 call comes in regarding a medical emergency.
Explore Resources
Here are some insights to help in your research journey


