
Join Princeton Blue and Appian at Booth 421
Feb 3-6, 2025
Orlando, FL
Booth 421


Join Princeton Blue and Appian at Booth 421
Feb 3-6, 2025
Orlando, FL
Booth 421

Presentations at SCOPE 2025
Using Gen AI for Content Extraction from ICFs to Ensure Compliance for Individual Site and Country to Meet Sample Destruction Timelines
🎙️Lisa Hersh Senior Manager, Regeneron
🎙️Anamika Sarkar, PhD, Intelligent Automation Lead, Global Development Solutions, Regeneron
⌚ Feb 4 2025 | 4:40 PM
Generative AI used to extract country and site-specific sample retention timelines from individual ICFs across sponsored clinical trials resulting in a user-friendly dashboard created to document differences in sample retention timelines and to ensure compliance with sample destruction for all subjects.
Using a sample destruction countdown feature, end users can see upcoming sample expiration dates and ensure sample destruction approvals and actions are completed and documented appropriately.
Optimizing Clinical Strategy with Innovative Technology in a Dynamic Organization
🎙️Amy McCormick, Senior Manager, Global Trial Optimization, Regeneron
⌚ Feb 6 2025 | 8:45 AM
During a period of significant growth and change, Regeneron’s Global Trial Optimization and Global Development Systems Teams implemented an innovative digital-first strategy to overcome challenges in trial feasibility, patient engagement, and site identification. Using an agile methodology and an Appreciative Inquiry model, the team identified workflow issues, designed a comprehensive solution, and delivered a flexible, scalable application.
This approach improved data quality, standardized deliverables, and optimized resources, providing a foundation for future business needs. The project highlighted the importance of collaboration, communication, and a strong change management strategy for successful technology adoption.
Our Experience in Life Sciences
Princeton Blue is a trusted leader in AI-powered process automation for pharmaceutical and biotech clients using Appian’s Low-code technology to deliver business outcomes in weeks.
We develop advanced workflow solutions that use Data Fabric to augment your existing systems such as Veeva, SAP and LIMS/ELN, and present a unified view of your enterprise data, use Private AI to turn that data into actionable intelligence, to deliver process automation across use cases in Clinical, Regulatory, Lab, and Manufacturing.
Princeton Blue is recognized by leading industry analysts for deep expertise Low-code technologies and decades of work experience in the Life Sciences industry. Some of the world’s largest pharmaceutical and biotech companies trust us to automate and intelligently transform their most strategic drug development processes. Let’s work together to accelerate your digital transformation journey.
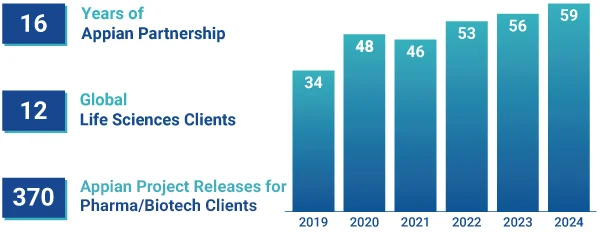
Projects Delivered
Clinical
Protocol Activity Schedule
Patient Eligibility
Clinical Supplies Job Readiness
Clinical Supplies Scheduling Tool
Study and Site Feasibility
Site Contact Information
Real-World Evidence
Monitoring Visit Report
User Access Management
Study Start-up
Clinical Planning
Standards Request Workflow
Patient Engagement Management
Integrated Evidence Planning
Clinical Complaint Management
Clinical Identity Access Management
Manufacturing
Asset Disposal
Temperature Excursion
Regulatory
EU Clinical Trials Regulation
Label Change Workflow
Dossier Change Control
Health Authority Correspondence
Regulatory Submission Planning
Regulatory Information Management
Lab
Lab Experiment Automation
Lifecycle Management
Lab Shared Services Request Processing
Medical Devices
Personalized Surgical Planning and Implementation
Regulatory Information Management
See How Merck Optimizes its Clinical Supply Chain
Combining workflow automation and data integration across the clinical supply chain to improve transparency, accountability, and the quality review and release process.
Hear from Merck on Clinical Automation
Mercks shares its automation journey around managing change in Clinical Data Management with Appian and Princeton Blue.
Solutions for Life Sciences
Clinical Complaint Management
Automate complaint handling, review, and categorization in clinical trials with Appian AI.

Labeling
Automate label management, stay compliant, and gain real-time insights with Process Automation, AI, and Data Fabric.

EU-CTR
Automate your EU-CTR compliance workflows for faster, error-free clinical submissions.

Regulatory Submissions
Streamline your regulatory submissions process for faster approvals and better compliance.

Clinical Supplies Shipping
Automate clinical supplies shipping with AI for consistent, efficient, on-time delivery.

Study Data Access Management
Securely manage user access requests for study data using AI & Process Automation.

Study Start-up
Automate the workflow and collaboration of the clinical study start-up process allowing for variations in business rules and regulatory requirements across countries.

Lab Experiment Automation
Automate lab operations to improve real-time visibility, reduce delays and drive reliable outcomes.
Drug Safety
Automate drug safety workflows for faster, consistent, and audit-ready reporting.

Dynamic Emergency Response
Streamline the triage between hospital nurses, doctors and ambulance EMT when a 911 call comes in regarding a medical emergency.
Build a Proof of Concept
Use our Innovation Lab to build a Proof of Concept before you invest in process automation technologies. Brainstorm with our team of experts and see what works best for you.
This is a very popular service and is used by many of our clients. We have delivered numerous Proof of Concepts for our clients. Contact us if you have a use case in mind and see how our Innovation Lab can help you.
